FDA Generic Education: What You Need to Know About Generic Drugs and Safety
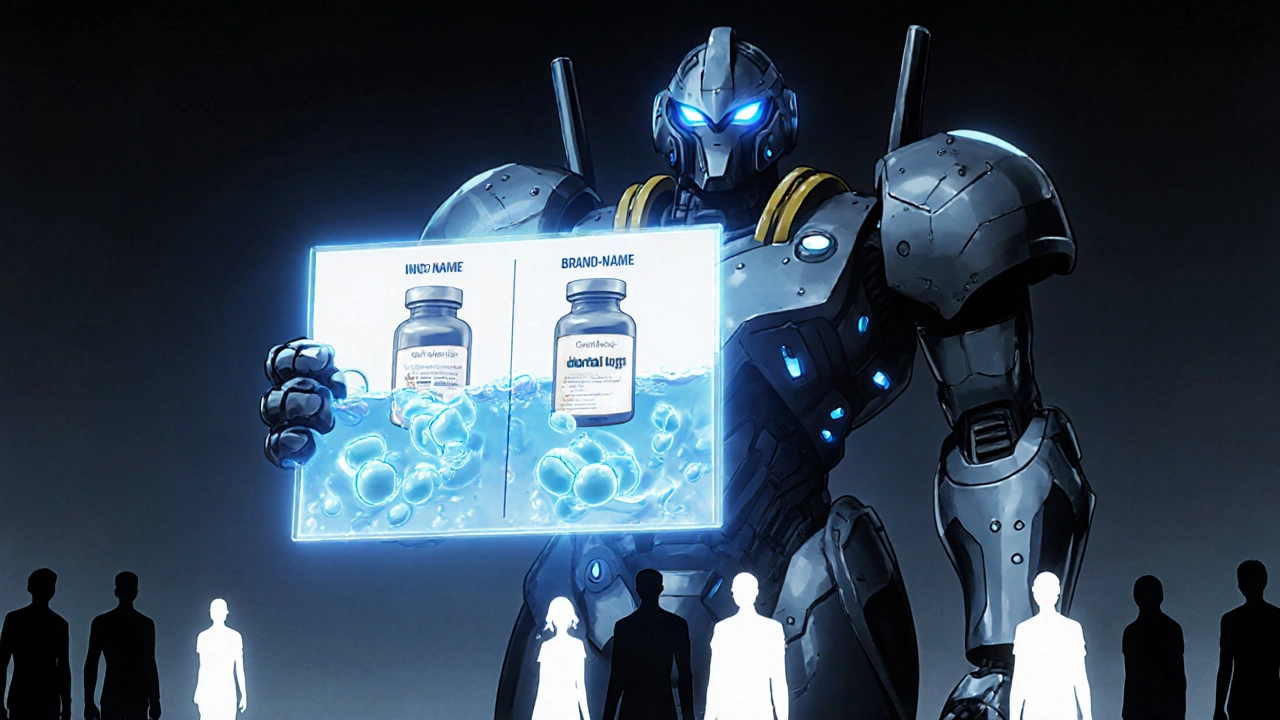
When you hear FDA generic education, the official information from the U.S. Food and Drug Administration about how generic drugs are approved and monitored for safety and effectiveness. Also known as generic drug approval guidelines, it’s not just paperwork—it’s the reason your $4 prescription works just like the brand-name version. The FDA doesn’t just approve generics because they’re cheaper. They require proof—real, measurable proof—that the active ingredient behaves the same way in your body. That’s called bioequivalence, the scientific standard that proves a generic drug delivers the same amount of medicine at the same rate as the brand-name drug. Without this, the FDA won’t let it hit the shelf.
Many people think generics are "almost the same," but that’s not true. They have to be identical in strength, dosage form, and how they’re absorbed. That’s why combination products—like pills with two drugs or inhalers with a device—face tougher testing. The quality assurance units, independent teams inside drug manufacturers that check every step of production to make sure nothing goes wrong don’t just sign off on paperwork. They audit equipment, review batch records, and test samples. If they’re not separate from production teams, the whole system breaks. And that’s why the FDA requires their independence—it’s not a suggestion, it’s the law.
What you don’t see matters just as much as what you do. Fake pills, counterfeit meds, and false allergy labels all tie back to gaps in FDA generic education. If you don’t understand how generics are tested, you can’t spot the red flags. That’s why posts here cover everything from bioequivalence challenges in complex drug forms to how FDA black box warnings, the strongest safety alerts the FDA can issue, warning of serious or life-threatening risks appear on generic labels. You’ll find real examples: why a generic version of a heart drug might need extra testing, how a simple pill log helps track if your medicine is working, or why a false penicillin allergy label can lead to worse antibiotics being used.
This isn’t about corporate jargon or regulatory theory. It’s about your health. Every time you choose a generic, you’re trusting the system to work. FDA generic education gives you the tools to know when that trust is justified—and when to ask questions. Below, you’ll find real-world guides from patients and experts who’ve seen what happens when these systems work… and when they don’t. No fluff. No marketing. Just what you need to know to stay safe and save money.